[試題] 97上 金必耀 普通化學甲上 期末考
課程名稱︰普通化學甲上
課程性質︰外系生/高中預修班普通化學
課程教師︰金必耀
開課學院:理學院
開課系所︰化學系
考試日期(年月日)︰2009/01/16
考試時限(分鐘):170 min (18:30~21:20)
(會視作答狀況調整,號稱最晚可以到十點,不過不需要這麼久)
是否需發放獎勵金:否
(如未明確表示,則不予發放)
試題 :
(NH_3 即氨,[NH_4]^(+) 即銨根,下列化學式排版規則同左例)
1.(10%) The 10 most common chemicals produced in the United States are as
follows: H_2SO_4, NH_3, CaO, O_2, N_2, C_2H_4 (ethylene), NaOH, Cl_2,
H_3PO_4, and NH_4NO_3. Which of these may be classified as salts?
Give the chemical name and draw a Lewis structure for each.
(跟 TA 確認的結果,最後是十個提到的分子的都要畫 Lewis structure)
2.(8%) Balance the following redox reactions occuring in neutral, acidic or
basic aqueous solution by the half-reaction method. As in homework, first
write the equation in ionic form and eliminate spectator ions, When you have
obtained a balanced equation, add spectators to give a balanced neutral
equation.
a. Br_2(aq.) + H_2SO_4(aq.) → HBr(aq.) + CO_2(g)
b. I_2(aq.) + [S_2O_3]^(2-) ─(KOH)→ I^(-)(aq.) + [S_4O_6]^(2-)(aq.)
c. [CN]^(-)(aq.) + [MnO_4]^(-)(aq.) ─(NaOH)→ MnO_2(s) + [CNO]^(-)(aq.)
d. [CrO_4]^(2-)(aq.) + [HSnO_2]^(-)(aq.) ─(KOH)→
[HSnO_3]^(-)(aq.) + [CrO_2]^(-)(aq.)
3.(10%) One family of recently discovered high-temperature superconductor is
typified by the trimetallic oxide YBa_2Cu_3O_7(s), a so-called "1-2-3
superconductor." Using the nobel gas rule for Y, Ba, and O, what is the
charge state of Cu in this compound?
4.(8%) Successful overlap using p orbital depends on their orientation with
respect to the covalent bond axis. By using symmetry arguments, show that the
overlap integral S is identically zero for overlapping an s orbital on one
atom with a p orbital on a neighboring atom that points perpendicular to the
internuclear axis. (That is to say, sigma-symmetry orbitals have zero overlap
with pi-symmetry orbitals.) It helps to make a sketch.
5.(9%) For each of the following molecules, use VSEPR to predict the bond
angles (and hybridization) of each atom bounded to more that one other atom,
and sketch the molecular shape.
a. GaH_3
b. SF_4
c. BeCl_2
d. SbCl_5
e. IF_5
6.(5%) Determine which of the molecules as shown in the previous question have
nonzero dipole moments.
7.(10%) For the molecule methyl fluoride (CH_3F, fluromethane), give valence
bond wave function describing the C-H bond and the C-F bond, and sketch the
overlapping orbitals for each.
8.(10%) The hydroxyl radical (OH) is a common intermediate in combustion
reactions, as well as an important, almost central, species in statospheric
chemistry. Analyze OH using MO theory, and predicts its magnetic properties.
9.(8%) For the redox reaction between K and ICl, identify the importance
frontier orbitals and sketch them. On the basis of your sketches, do you
expect to see a greater yield of KI or KCl?
10.(8%) Shinning 266-nm light on HBr causes dissociation into H and Br atoms.
What MOs are involved in this transition? Given the bond energy of HBr,
D_0 = 3.751 eV, what is the kinetic energy of the atom after dissociation?
11.(10%) In the N_2 molecule, which transition, sigma^2p to pi^star, or pi
to pi^star, will produce a greater change in the bond length? How is this
difference reflected in the absorption bands for these two transitions?
12.(10%) The vibrational of degree of HCl is only one. However, there is a
tiny splitting in the IR specturm of HCl as shown in the figure below. What
is the origin of this splitting? Rationalized your answer based on the
vibrational frequency = [(force constant/reduced mass)^(1/2)]/2pi,
in particular, you have to discuss the dependence of vibrational frequency
on force constant and reduced mass (= [m_1 * m_2] / [m_1 + m_2]) and their
relative importance.
http://hyperphysics.phy-astr.gsu.edu/Hbase/molecule/imgmol/hclspec.gif
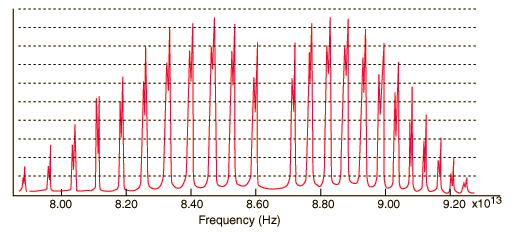
(原始圖當然不是這張,重點是每個 peak 都有 splitting)
--
當我們在遠方相遇,我必能清楚看見
你已為我開燈點亮整個宇宙的黑暗
--
※ 發信站: 批踢踢實業坊(ptt.cc)
◆ From: 61.230.31.7
推
01/18 15:00, , 1F
01/18 15:00, 1F
※ KenYang0531:轉錄至看板 civil98 01/13 23:34